Healthcare Economist |
| Which strategies are payers and employers using to cover orphan drugs? Posted: 06 Jun 2021 11:06 PM PDT This is the question posed by a recent article by Lopata et al. (2021). The authors surveyed 26 payers (e.g., leaders of national, regional, local, Medicaid, and Medicare plans; payer components of integrated delivery systems; and pharmacy benefit managers (PBMs) and 11 employers (e.g., large employers, employee benefit consultants, employer coalitions). Respondents were informed that orphan disease was defined as "a condition that affects fewer than 200,000 people in the United States." Most payers used resinurance (42%), a gene therapy carve-out (31%) or orphan drug carve-outs (23%). While employers also favored the use of reinsurance (55%), use of carve outs was rare and shifting benefits from medical to pharmacy coverage (36%) or benefit exclusions (27%) or third party subscription models (27%) were more common. 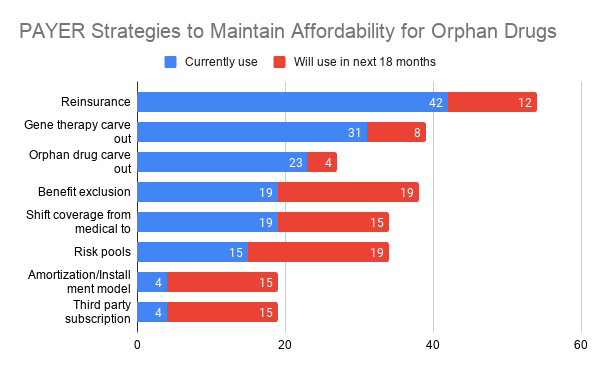 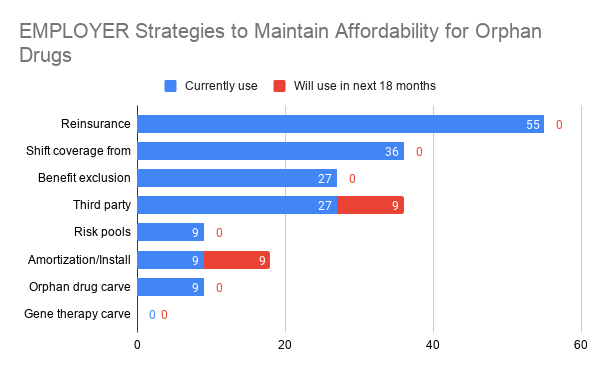 Are limited distribution networks the answer? Payers didn’t think so.
Another key question is whether to cover health care provider (HCP)-administered orphan drugs under a medical or a pharmacy benefit. There are reasons that justify both options.
The development of new treatments for rare diseases is one we should welcome, as these patients need treatment. At the same time, payers and employers are likely to continue exploring creative strategies for making coverage of these treatments affordable.
|
| You are subscribed to email updates from Healthcare Economist. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
No comments:
Post a Comment