| The Biden administration has started putting meat on the bones of a sweeping new measure protecting Americans from surprise medical bills. Regulations released yesterday are just a start — but they provide some new details about how patients must be protected from these "surprise" bills when they unavoidably get care from an out-of-network emergency room or doctor. "If we're going to keep people from being blindsided with some of these charges, we need to make sure it's straightforward how everyone does this," Health and Human Services Secretary Xavier Becerra told reporters in a call yesterday afternoon.  Health and Human Services Secretary Xavier Becerra testifies before the Senate Finance Committee last month. (J. Scott Applewhite/AP) | The administration has six months to finalize regulations for the "No Surprises Act."The bill, passed by Congress in December, stands as an extraordinary example of how Congress can still pass bipartisan legislation if there's overwhelming consumer — and industry — support behind it. After 10 years of bitter political battles over the Affordable Care Act, lawmakers closed out the decade with the bipartisan, widely supported legislation sheltering patients from sky-high bills when they're forced to get care from a provider that isn't contracted for in-network care with their health insurance plan. These bills typically result when someone experiencing an emergency must seek care from the closest hospital, or when they visit an in-network hospital but receive care from an out-of-network provider through no fault of their own. Over the last few years, journalists have chronicled enormous surprise medical bills, sometimes landing people in tens of thousands of dollars of medical debt. The effort to pass surprise billing protections was never guaranteed success; in the months-long process, there were many moments in which the whole thing threatened to crumble. The health insurance industry lined up behind one bill while doctors lined up another, as lobbyists on both sides fought to influence how payment for these bills would be worked out.  (Angela Weiss/AFP) | Yesterday's regulation gave more details on how patients should be protected from surprise bills.The rule, which is open for comment until Sept. 1, spells out how medical services should be determined as emergency or non-emergency for surprise billing protections. It warns that insurance plans can't retroactively decide an emergency visit wasn't urgent. It also issues some clarifications around how to determine median in-network rates when pegging out-of-network payments to them. The Department of Health and Human Services also released a standard notice form to be used by hospitals in letting patients know they're receiving out-of-network care. | Krutika Amin, associate director for the Kaiser Family Foundation's Affordable Care Act program: | 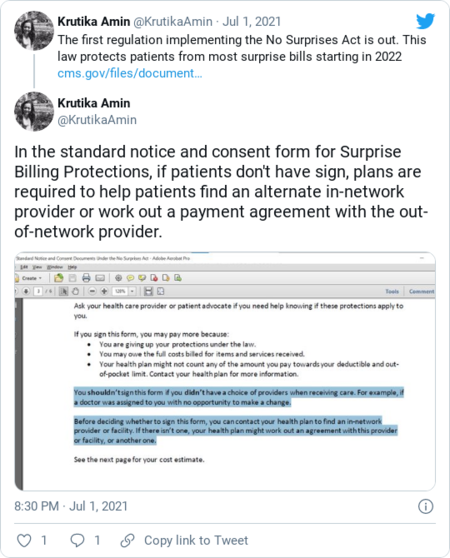 | | | Lobbyists are still working hard to influence the regulations.Everyone agrees on this: Patients can only be charged the normal, in-network rate for a surprise medical bill, and nothing more. "No one should ever be threatened with financial ruin simply for seeking needed medical care," Labor Secretary Marty Walsh said in a statement. As expected, patient advocacy groups applauded yesterday's interim final rule. "The No Surprises Act marked a major breakthrough for patients seeking relief from surprise bills, and we are thoroughly reviewing the rule released today to ensure the intent of Congress is upheld," the American Heart Association said in a statement. But behind the scenes, insurers and provider groups have still been trying to influence HHS as it works out the specific mechanics of how the legislation will work. Under the No Surprises Act, the two parties must spend 30 days trying to negotiate the rest of the bill. If they can't work it out, an independent arbiter steps in. Both sides must submit a final offer, and the arbiter decides which offer is most reasonable. But there's been a lot of head-butting over which types of payments the arbiter may take into consideration when making a final call. More details on exactly how arbitration should work are forthcoming; over the next few months, HHS will be working on additional regulations spelling out procedures for that part of the No Surprises Act. Note to readers: The Health 202 won't publish on Monday, July 5, in observence of Independence Day. Have a lovely long weekend and we'll see you back here Tuesday. | | | Ahh, oof and ouch AHH: The Johnson & Johnson vaccine is effective against the delta variant.The company is highlighting a small study in which blood samples obtained from eight inoculated people showed that Johnson & Johnson's single-dose shot generated a strong immune response against the delta variant, the New Brunswick, N.J.-based company said. (The results have not been peer reviewed.) Earlier clinical trials had shown the vaccine offered 66 percent protection against symptomatic infection, our Katerina Ang reports. "We believe that our vaccine offers durable protection against COVID-19 and elicits neutralizing activity against the Delta variant," Paul Stoffels, chief scientific officer at Johnson & Johnson, said in a news release. "This adds to the robust body of clinical data supporting our single-shot vaccine's ability to protect against multiple variants of concern." The study offers more hope amid a summer surge of the highly contagious strand. "The data so far indicates that the three U.S.-approved vaccines offer effective protection against all known variants of the virus. Analysis by British health authorities, drawing on data from a large pool of people, indicates that the Pfizer-BioNTech messenger RNA vaccine provides 96 percent protection against hospitalization from the delta variant, which was first detected in India," Katerina writes.  (Photo by FREDERIC J. BROWN/AFP via Getty Images) | OOF: The White House is launching "surge response" teams to delta variant hot spots."These are dedicated teams working with communities at higher risk for, or already experiencing, outbreaks due to the spread of the delta variant and their low vaccination rate," White House coronavirus coordinator Jeff Zients told reporters at a news briefing. The announcement comes as the more transmissible delta variant is fast taking over the United States. It now represents one-quarter of confirmed cases and is the dominant variant in Arkansas, Colorado, Missouri and Utah.  A vaccine clinic in Missouri. (Nathan Papes/Springfield News-Leader/AP) | "The White House-coordinated teams will include a mix of virtual support and on-the-ground personnel, helping deploy additional supplies as requested by local officials, such as testing or therapeutics. Staff will come from the CDC, the Federal Emergency Management Agency and the Office of the Assistant Secretary for Preparedness and Response in the Department of Health and Human Services. The White House also may ramp up paid promotions about the benefits of vaccination in areas that officials deem high risk," The Post's Dan Diamond reports. The variant will pose the greatest risk to communities where vaccination rates are low. Centers for Disease Control and Prevention Director Rochelle Walensky warned that there are 1,000 U.S. counties where fewer than 30 percent of residents are vaccinated. Yet there's lots to celebrate over the 4th of July weekend, officials stressed. "This weekend, millions of Americans will be able to get together -- back together, not just with their families and close friends for small backyard cookouts, but with their community for larger festivals, parades, and fireworks, celebrating our country's July 4th Independence Day and the progress we have made against the virus together," Zients said.  Coronavirus task force chief Jeff Zients. REUTERS/Kevin Lamarque/File Photo | OUCH: House Democrats are trying to scrap an antiabortion measure in foreign aid spending.During a House Appropriations Committee markup of a foreign spending bill, Republicans repeatedly tried to insert a policy rider, known as the Helms Amendment, which bans U.S. foreign aid from funding abortions. This rare but long-standing agreement has for decades allowed Congress to pass appropriations bills without getting tangled up in perennial fights over abortion. But Democrats — who over the past few years have increasingly backed away from Helms and other bans on taxpayer funding for abortions — defeated introduction of the measure. Voting along party lines, the House Appropriations Committee advanced a foreign aid spending bill that does not include the Helms Amendment. But Senate Republicans may insist on adding it back in. The question is whether the "language can survive contentious negotiations in the Senate where lawmakers who support abortion rights will need to contend with the Republican filibuster, which could lead to the watering down of the abortion access provisions that House Democrats are proudly championing this budget cycle," Roll Call's Rachel Oswald reports.  An abortion-rights protest in Quito, Ecuador. (AP Photo/Dolores Ochoa, File) | | | | On the Hill The bipartisan infrastructure deal struck last week left out long-term care spending.Biden's initial American Jobs Plan called for $1.7 trillion in new spending, including $400 billion in funding for long-term care, with an eye to helping the rapidly aging boomer population get care at home instead of in nursing facilities. But a bipartisan infrastructure deal for $579 billion in new spending leaves out long-term care funding, along with a slew of other Democratic priorities not traditionally considered part of core U.S. infrastructure, The Post's Ashlyn Still and Daniela Santamariña report. The new deal also pares down money aimed at improving U.S. water infrastructure. Biden had initially proposed spending $111 billion to upgrade aging water infrastructure and replace lead pipes. The bipartisan deal cuts water infrastructure spending in half, to $55 billion. Exposure to lead-contaminated water can lead to health and learning problems, especially for children. Liberal Democrats have been pushing a companion bill focused on social programs, which is unlikely to garner Republican support. Democrats hope to pass the bill with a simple majority through reconciliation, rather than through the traditional legislative process, which usually requires 60 votes. | | | More in coronavirus news - The Federal Emergency Management Agency has changed the rules in its pandemic funeral assistance program to make it easier for family members of those who died of covid-19 early in the pandemic, before testing was commonplace, to access funds. They will be able to submit for reimbursement even if the death certificate does not list covid-19 as the cause of death, Politico's Erin Banco reports.
- Europe has launched digital vaccination cards aimed at facilitating free movement across borders. But the certificates have already faced pushback, including from vaccinated would-be travelers who received shots not yet been approved by European Union regulators, The Post's Miriam Berger reports.
 The welcome screen of the new European Union covid-19 certificate. (Benjamin Girette/Bloomberg News) | - Health officials are worried that Fourth of July festivities could trigger coronavirus outbreaks, especially in states such as Missouri where vaccinations rates are low, the Associated Press reports.
| | | Elsewhere in health care Companies are offering genetic screening services aimed at selecting "healthier" embryos.In a report published Wednesday in the New England Journal of Medicine, a group of researchers raised concerns about the predictive power of these screening tests and unintended consequences. Genetic tests have gone from identifying single disease causing genes, such as the one for Tay-Sachs disease, and can now be used to analyze small genetic differences across a genome. This is used to calculate a score estimating someone's risk for certain traits or diseases, such as cancer. But some scientists question how accurate these screenings are at predicting differences between embryos from the same parents. The screenings also raise ethical questions. As recently as December, Genomic Prediction advertised its ability to screen for intellectual disability. "The report underscores the concern among geneticists, legal scholars, and ethicists that applying genetic research to develop risk scores and select embryos might be imperfect and less robust than patients and companies think," Stat News's Claudia Lopez reports. An advocacy group is targeting Democratic lawmakers who did not support drug pricing reform.Patients for Affordable Drugs, a national patient organization, is launching an ad campaign urging lawmakers to support the drug pricing bill H.R.3, which would allow Medicare to negotiate directly with drugmakers. The latest round of ads will target lawmakers who have refused to endorse the latest version of the drug pricing bill, including Reps. David Valadao (Calif.), Scott Peters (Calif.), Richard Hudson (N.C.), and Kurt Schrader (Ore.). | | | Sugar rush | | | |













No comments:
Post a Comment