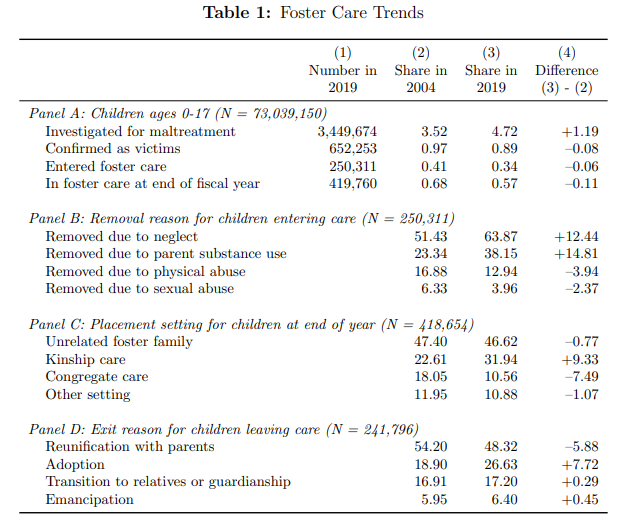
| The Economics of Foster Care Posted: 15 Apr 2022 04:05 PM PDT This is something I knew very little about and thus Bald et al. (2022) NBER working paper was fascinating to read. They describe the key tension of foster care: failing to put at risk children in foster care may expose them to abuse or neglect; however putting too many at risk children into foster care leads to a dismantling of families. Some interesting excerpts are below: - Prevalence/Incidence: “5 percent of US children are placed in foster care at some point during childhood, and similar rates are found in other countries…. In 2019 [Child Protective Services] CPS investigated nearly 3.5 million children for maltreatment (nearly 5 percent of all children), and classified about 652,000 children as victims (nearly 1 percent of all children)…a remarkable 37 percent of all children experience a child welfare investigation, and 12 percent of children are identified as maltreatment victims, including 18 percent of Black children and 16 percent of Native American children”
- “Orphan trains”: “At the turn of the last century, ‘orphan trains’ transported thousands of neglected children from cities to live and work on farms”
- High rates of depression. “In comparison to similar children who did not spend time in foster care, foster children have rates of depression and anxiety that are seven times higher”
- Incarceration. “Close to 20 percent of the United States prison population were in foster care as a youth”
- Employment outcomes. “Those who turn 18 in foster care report 50 percent lower earnings and employment rates that are 20 percentage points lower by age 26 compared to a sample of young adults with similar levels of education as many as one-third experience homelessness”
- Foster care cost. “Child welfare agencies currently spend over $30 billion each year on child protection, with approximately half of their funding provided by the federal government… average monthly foster care payment was just over $500, which is twice the amount of the average Temporary Assistance for Needy Families benefit “
- Racial disparities. “Racial disparities occur at nearly every foster care decision point. Black children are reported to child protective services more frequently than White children, and reports involving Black children are more likely to be confirmed as maltreatment” Cumulative foster care rates are 10%-12% for Blacks, and 11-15% for American Indian/Alaska Native children compared to 5% for White children.
- Geographic disparities. Lowest rates are <3 per 1,000 children in New Jersey, Utah, and Virginia compared to >15 per 1,000 children in West Virginia, Montana, and Alaska.
- Risk factors: These are fairly obvious: poverty, substance abuse (especially in recent years opioid abuse).
- Performance based contracts. Some states are experimenting with linking child outcomes to foster care payments. A key issue is, would this incentivize foster families to try to avoid the most at risk kids?
Foster care trends |
| Why is investing in drugs to treat rare diseases an attractive investment for drug companies? Posted: 14 Apr 2022 09:12 PM PDT If you were a drug manufacturer, you naturally would want to focus your research on more common, highly prevalent diseases. If there are more patients with the disease, that means more drugs that can be sold. In recent years, however, the number of so-called “orphan drugs” has increased. In the 1970s, only 10 orphan drugs were approved. Between 1983 until 2019, there have 564 orphan drugs that had been approved by the FDA. Why the change? The primary reason was the passage Orphan Drug Act (ODA), which created financial incentives to encourage companies to develop new drugs for rare disease. What were these incentives? I list a number of them below, many summarized from a recent ICER white paper on rare disease. - Longer market exclusivity. Manufacturers receive 7 years of market exclusivity for orphan drugs compared to only 5 years for non-orphan drugs. .
- R&D Tax credits. Clinical trial costs incurred to develop an orphan drug can be partly offset with a 25% tax credit which is bankable for future years of a company is not yet profitable.
- FDA user fee waiver. Prescription Drug User Fee Act (PDUFA) fees—which generally cost $3.2 million—are waived for orphan drugs.
- Research grants. Manufacturers developing orphan drugs are eligible for research grants from the Office of Orphan Products Development (OOPD).
- 340B exemptions. Orphan drugs are exempt from the requirement to give special discounts to pharmacies associated with 340B covered entities. Recent court rulings have indicated that orphan drugs are exempt from the 340B discount requirement even for non-orphan indications of any drug with at least one orphan indication.
- FDA Accelerated approval. In 1992, FDA instituted an accelerated approval pathway to expedite drug approval for serious conditions. While this approval process was not specific to orphan drugs, 1 in 7 orphan indications has received accelerated approval, largely due to the lack of any alternative treatment.
|
No comments:
Post a Comment